
Cordlife Clients
We strive to provide you with greater value, trust, and convenience.
FAQ for Expectant Parents
Over 40,000 cord blood transplants have been done around the world, and cord blood is quickly becoming a good alternative to bone marrow stem cells.1 Many promising research and clinical trials are taking place in the area of stem cell transplants.
The FAQs below present most of the commonly asked questions have about cord blood banking and other related questions. Find out more about cord blood banking and how Cordlife can help play an important part to ensure holistic protection and assurance when it comes to protecting your child's health.
To view a topic, click on it and find the answer.
Don’t see the answer to your question? Give us a Call our Hotline at (02) 8470-1735 or (02) 8332-1888 or or email us at ph.info@cordlife.com
Reference:
1. Hal E Broxmeyer. The history of cord blood transplantation/biology & perspective for future efforts to enhance the field. https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/393/The-history-of-cord-blood-transplantation-biology-perspective-for-future-efforts-to-enhance-the-field. Accessed August 12, 2020.
What is cord blood?
Cord blood is the blood that remains in the umbilical cord after a baby is born and after the umbilical cord is cut. During pregnancy, the umbilical cord serves as a lifeline between mother and child. Following a baby’s birth, the cord blood may offer hope to both the child and family members. Cord blood is a rich source of lifesaving stem cells known as haematopoietic stem cells (HSCs).
What are cord blood stem cells?
Cord blood stem cells, which are also called haematopoietic stem cells (HSCs), are in charge of making new blood and repairing the immune system. These cells can be found in a baby's umbilical cord after birth and have the unique ability to differentiate into various cell types, as illustrated in the diagram below:
- Red blood cells - transport oxygen
- White blood cells - produce antibodies and fight bacteria
- Platelets - assist in blood clotting
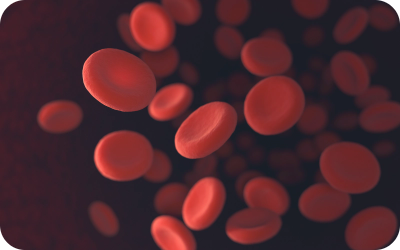
Carry oxygen to all cells in the body
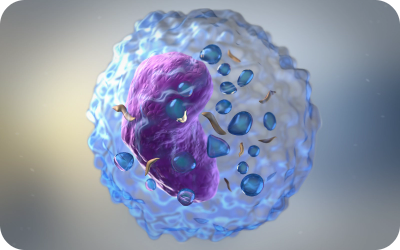
Fight infections
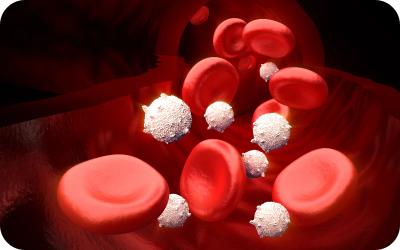
Helps blood to clot in the event of an injury
Why should I save my baby’s cord blood stem cells?
There are several benefits to storing your baby’s cord blood stem cells, including:
- A perfect match for autologous transplantation (where the donor and recipient are the same individual).
- Having a readily available supply of haematopoietic stem cells rather than conducting a costly and time-consuming national or international search during a time-critical situation.
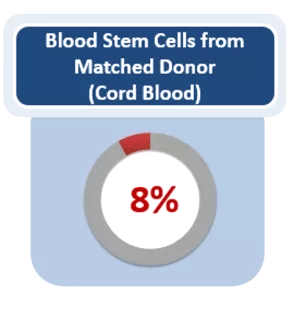
- Lower risk of Graft vs. Host Disease (GvHD), a condition in which the transplanted tissue attacks the patient’s own tissue.
- Non-invasive collection procedure that is painless and risk-free for both mother and child
- Cord blood stem cells are younger, have a higher rate of engraftment, and are more tolerant to tissue mismatches than other sources of stem cells, such as bone marrow.
Lower risk of Graft vs Host Disease (GvHD) after stem cell transplant
What can cord blood stem cells do?
Cord blood stem cells can:
- Replace and regenerate damaged or diseased bone marrow.
- Treat blood cancers, solid tumours, and immunodeficiency disorders.
- Correct genetic defects (sibling or allogeneic transplantation).
- They have the potential to be used for cellular therapy and regenerative medicine.
How does a cord blood stem cell transplant work?
The goal of a stem cell transplant is to rebuild a patient’s blood and immune system.
In the case of a cancer-related transplant, for example, chemotherapy and radiotherapy will be required prior to the infusion of cord blood stem cells. The cord blood stem cells will be injected intravenously into the patient’s bloodstream, and will migrate to the patient’s bone marrow. Haematopoietic stem cells found in umbilical cord blood will differentiate into three types of blood cells—red blood cells, white blood cells, and platelets— initiating the regeneration of the patient’s blood and immune system.
What are some of the diseases that cord blood stem cells can treat?
Cord blood stem cells can potentially be used as a standard treatment for over 80 diseases, including leukaemia, lymphoma, thalassemia, and neuroblastoma, to name a few. For the full list of treatable diseases, click here .
How will storing cord blood stem cells benefit my family?
In addition to being a perfect match for your baby, cord blood can also be used by family members. Unlike bone marrow, which requires a perfect match between donor and patient, the likelihood of finding a cord blood match among family members is higher. Also, the chances of finding a match within the family go up with each additional child whose cord blood is stored.¹
1 Parent’s Guide to Cord Blood FAQ. Parent’s Guide to Cord Blood Foundation website. https://parentsguidecordblood.org/en/faqs. Accessed November 11, 2019.
How does my obstetrician collect umbilical cord blood from my baby?
Your baby’s umbilical cord will be clamped and cut immediately after birth. After making sure your baby is healthy, your obstetrician will collect the blood from the umbilical cord by sticking a needle into the vein and letting the blood flow into a blood bag.
This procedure is non-invasive, painless, and risk-free for both mother and child. The procedure lasts about three minutes and can be performed during a vaginal or caesarean delivery.
Please note that the final decision on whether or not to collect cord blood will always be made by your caregiver, whose priority is to ensure the safety of you and your baby.
What happens after my baby's cord blood is collected?
After your baby’s cord blood is collected, our courier will pick it up and deliver it to our stem cell processing and cryopreservation facility within 24 hours.
Our biotechnologist will begin isolating the stem cells from your baby's cord blood in the laboratory. This is a very important part of cord blood banking, as it determines the number of stem cells that can be extracted or recovered from the cord blood.
After the stem cells have been separated and stored in our FDA-approved cryogenic storage pouch, they are put in Smart-MaxTM, a fully automated cryoprotectant infuser, mixer, and cooling device that helps get the cells ready for cryopreservation. Smart-Max TM makes sure that the cryoprotectant is injected at a constant temperature that has already been set. This keeps your baby's stem cells alive.
1 Cordlife umbilical cord blood processing data as of 14 March 2019
How is my baby's cord blood stored?
Your baby's umbilical cord blood is stored within a US FDA-approved cryogenic storage pouch made of a special material that is specifically designed to withstand cryogenic temperatures.
The pouch has two main segments (20% and 80%) that are attached integrally, and two test segments that are also integrally attached.
The integral segments allow for additional testing on the associated unit to ensure that no samples are mixed up and that the cord blood remains viable. These tests are typically performed prior to a transplant.
Having dual integrated segments addresses the possibility of future stem cell expansion. This means that when stem cell expansion is available commercially, you will be able to withdraw a portion of the stem cells for expansion while keeping the remainder in storage.
How long can my baby's cord blood stem cells be stored?
Research shows that cord blood that has been processed according to international standards and stored at cryogenic temperatures between -135°C and -190°C can be kept viable indefinitely. ¹
1 Cells for life website. https://cellsforlife.com/how-long-can-cord-blood-stem-cells-be-stored/. Accessed November 25, 2019.
Can I store my baby's cord blood if I test positive for Hepatitis B?
You can still continue to store your baby's cord blood (with additional consent given to Cordlife), or you can choose to discard the cord blood.
This is because Cordlife runs two different Hepatitis B virus (HBV) tests on maternal blood, namely:
- Hepatitis B Surface Antigen (HBsAg)
- Hepatitis B Core Antibody (Anti-HBc (Total))
The attending transplant doctor will usually decide whether to use a cord blood unit whose mother's blood tested positive for Hepatitis B for transplant based on a number of factors that are specific to the patient. These factors include the cord blood unit that will be used and the availability of other HLA-matched donors.
What happens after the full 18-years of storage?
The ownership of the umbilical cord blood unit will be transferred over to your child once he/she reaches the legal adult age of 18 years and upon the renewal of the agreement/contract. Your child will be prompted to continue storage thereafter at the prevailing fees.
What is the cost of banking my baby's cord blood?
Contact us (02) 8470-1735/8332-1888 or +63 949 889 3603 or you can email us ph.info@cordlife.com to make an appointment with our Cord Blood Banking Consultant.
How do I sign up for cord blood banking?
Contact us (02) 8470-1735/8332-1888 or +63 949 889 3603 or you can email us ph.info@cordlife.com to make an appointment with our Cord Blood Banking Consultant.
*If your baby is due in the next 4–6 weeks, we strongly advise you to contact us as soon as possible to enrol so that all necessary steps are done before your baby arrives.
If I bank my baby's cord blood privately, am I depriving the public registry of a sample?
Currently, only a small percentage of births in the world result in entries in a public cord blood registry. Donor eligibility is also greatly reduced by maternal blood virus exposure, tattoos, and international travel. The limited cord blood supply in most public banks is mainly due to a lack of public funding and not a direct result of private banking.
But we are sure that no one should throw away this valuable resource, because someone could benefit from its healing power. If you want to bank, think about your options and pick the best one for you. If you do not want your baby’s cord blood to be stored privately, please donate it.
What is the point of private banking in the first place? Isn't this something that should be shared?
Whether to bank privately or in a public bank is a very personal choice that expectant parents make after getting all the information they can. Given that the cause of most cancers is unknown, and given the rapid advancements in the field of stem cell therapy, families may be able to benefit from the private storage of their own stem cells. Based on what we know now, stem cells that are properly frozen and stored can stay alive for more than a lifetime. If every mother knew about both private and public cord blood banking options, there would be enough cord blood in public registries.
Isn't saving one child's cord blood enough? Is it necessary for me to bank the cord blood of my second child as well?
Cord blood stem cells, like each child, are genetically unique. Parents might want to store each child's cord blood to make sure that it is a perfect genetic match. This may also increase the likelihood of a suitable match between family members.
In identical twins, it is still critical to save as many stem cells as possible, and cord blood should be collected for each twin. In general, the collection volume for each twin is lower, so collecting from both babies helps to ensure an adequate stem cell yield for transplants if necessary. Even if the twins are identical, the cord blood is banked separately. However, such pregnancies are considered “high risk”, and your obstetrician or caregiver will always prioritise the mother and baby over cord blood collection, so cord blood may not be collected.
Am I exposing my baby to health problems by opting for cord blood collection rather than having the blood milked back to him/her?
A common misconception is that if parents choose cord blood collection, the baby will be deprived of this blood, potentially exposing the child to anaemia in the future. There is no scientific evidence that returning the blood to the baby benefits the baby’s health. The medical community now believes that the baby does not require the blood and that milking the cord blood back to the baby has no effect on the baby's risk of developing anaemia in the future.
I don’t understand why I need to have my blood drawn and tested; aren't my doctor's results sufficient?
We will need to collect maternal blood samples on the day of birth (either before or after the baby is delivered). Since infections can happen up to seven days before birth, it is very important to test the mother's blood again. This practice is recommended by the AABB and FACT-Netcord to ensure that the cord blood sample stored is safe for a future transplant.
How do I know that the storage of my baby's cord blood sample will continue beyond the company's lifespan?
It is critical to understand that the company you choose to bank with can at the very least guarantee that the sample will be cared for for the duration of the service agreement. No company can promise to last forever, but we have taken steps to make sure we have enough money in the bank to store all of our clients' samples in the future. As a publicly listed company, our financial status is shared publicly. You can download a copy of our recent annual report here: https://cordlife.listedcompany.com/. Furthermore, if the company assigns the business elsewhere, we ensure continuity of storage by transferring your sample to another facility nominated by us at no cost.
